Silicon Chemistry and Meteoric Smoke
Middle/Upper Atmosphere Laboratory, Past Research.
New particles in the atmosphere: two non-classical examples (NERC standard grant NE/E005659/1)
This project (2007-2010) addressed the spontaneous formation, growth and atmospheric impacts of two types of particles: iodine oxide particles (IOPs) in the marine boundary layer, and meteoric smoke particles (MSPs) in the mesosphere.
Meteoric Smoke particles (MSPs)
An important component of this part of the project was to develop the first model of silicon chemistry in the upper atmosphere. The kinetic studies on reactions of Si+, SiO+, Si and SiO (Fig. 1) showed that silicon, freshly ablated from meteoroids, is quickly converted to very stable silica molecules. 1,2,3 Hence, SiO2 is expected to be a major building block of MSPs, along with Fe and Mg oxides.
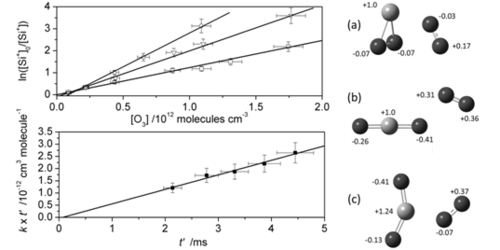
Figure 1. Left: Determination of the rate coefficient for the Si+ + O3 reaction. Upper panel: logarithmic, normalized integrated Si+ signal vs. O3 concentration (as determined from flows and pressure readings), for flight times of 2.1 ms (squares), 3.3 ms (triangles) and 4.5 ms (circles). The first order rate coefficient for a particular flight time is given by the gradient of the corresponding plot. Bottom panel: first order rate coefficients vs. flight time. The slope of this plot gives k(Si+ + O3). Right: Optimised geometries of the clusters of O2 with: (a) SiO2+(2A2); (b) OSiO+(2Π); and (c) SiO2+(4B1), calculated at the CAM-B3LYP/6-311+g(2d,p) level of theory. The light- and dark coloured atoms are Si and O, respectively. The signed number next to each atom is the Mulliken charge.
The formation kinetics of MSPs were then studied, starting with the magnetic-dipole driven growth of fractal Fe-containing particles.4 We also developed the first successful technique to generate olivine-type MSPs in the gas phase, where the Mg/Fe ratio can be precisely controlled (Figs. 2 and 3).5
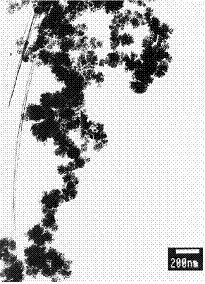
Figure 2. Transmission electron micrograph of a typical fractal-like particle aggregate formed from a vapour mixture of Fe, Mg and Si precursors.
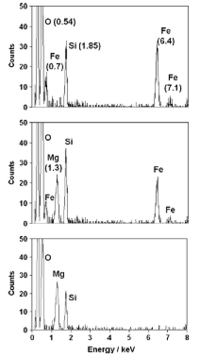
Figure 3. EDX spectra of particles sampled from three particle production experiments in which no Mg precursor vapour was present (top panel), no Fe precursor vapour was present (bottom panel), and in which all three precursor vapours were present in the flow reactor (middle panel). Characteristic element peaks are indicated at the respective electron energy values.
The ice-nucleating properties of Fe2O3 nano-particles were then studied in the AIDA chamber (Karlsruhe) (Fig 4). We observed efficient nucleation on 15 nm particles at temperatures down to 180 K,6 which we believe are the smallest particles where ice nucleation has ever been observed. Fe-Mg-Silicate nano-particles were also generated using sol-gel synthesis, and shown to react very efficiently with concentrated H2SO4 even at the low temperatures of the lower stratosphere (Fig. 5 and 6).7
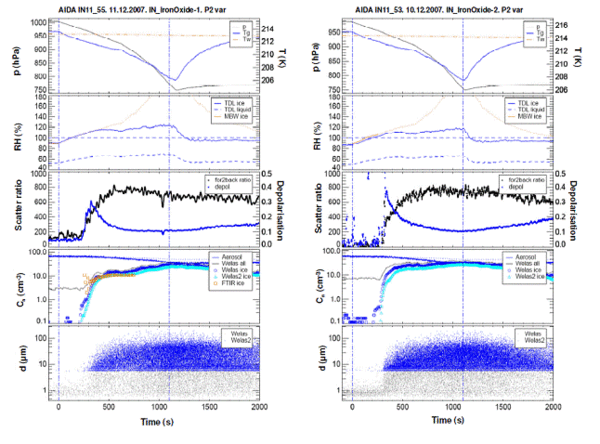
Figure 4. AIDA chamber ice nucleation data for Fe2O3 powders: left-hand side is for laboratory generated aerosol and right-hand side for a commercial sample. The initial chamber air temperature was 214 K as indicated by the blue line at t = 0 s in the top panel of each data set.
Figure 5. Optical microscope image of synthesised silicate particles used in the acid solution-powder experiments.
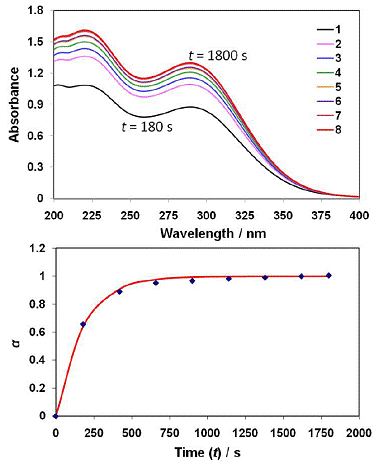
Figure 6. The top panel shows a time series of UV-visible spectra taken from a 75%Wt H2SO4 solution at 273 K, to which amorphous fayalite powder had been added. The time at which each numbered (consecutive) spectrum was taken is indicated in the bottom panel, which is a plot of the ratio of measured absorbance at the 289 nm (Fe[SO4]−2 ) peak to the maximum (final) absorbance at time (t).
The final part of the project was to study the transport of MSPs from the upper mesosphere down to the earth’s surface, using the Met Office Unified Model (HadAM 3). This work shows that MSPs remove significant amounts of H2SO4 in the stratosphere, before entering the troposphere where the greatest density of MSPs is deposited in Greenland (in accord with ice core measurements). The UM was also run to simulate under the conditions of the last Ice Age, revealing that the greatest deposition of cosmic material occurred over North America and Antarctica.7
References
- Gómez Martín et al., Kinetic Studies of Atmospherically Relevant Silicon Chemistry: Part I: Silicon atom reactions, PCCP, 2009, 11, 671-678.
- Gómez Martín et al., Kinetic Studies of Atmospherically Relevant Silicon Chemistry: Part II: Silicon monoxide reactions, PCCP, 2009, 11, 10945-10954.
- Gómez Martín and Plane, Kinetic Studies of Atmospherically relevant silicon chemistry. Part III: Reactions of Si+ and SiO+ with O3, and Si+ with O2, PCCP, 2011, 13, 3764-3774.
- Saunders and Plane, The Formation and Growth of Fe2O3 Nanoparticles from the Photo-Oxidation of Iron Pentacarbonyl, J. Aerosol Sci., 2010, 41, 475-489.
- Saunders and Plane, A Photo-Chemical Method for the Production of Olivine Nanoparticles as Cosmic Dust Analogues, Icarus, 2011, 212, 373-382.
- Saunders et al., An Aerosol Chamber Investigation of the Heterogeneous Ice Nucleating Potential of Refractory Nanoparticles, Atmos. Chem. Phys., 2010, 10, 1227-1247.
- Saunders et al., Interactions of meteoric smoke particles with sulphuric acid in the Earth’s stratosphere, Atmos. Chem. Phys., 2012, 12, 4387–4398.
