Calcium Chemistry in the Mesosphere/Lower Thermosphere (MLT)
Middle/Upper Atmosphere Laboratory, Past Research. 2006-2009
In the upper mesosphere/lower thermosphere (MLT) thin layers of metal atoms are present between 80 and 105 km due to the ablation of interplanetary dust particles (meteoroids) entering the Earth's atmosphere. These metal atoms have been observed by ground-based lidar and the calcium layer has been found to have some unexpected properties.1 Ca is extremely depleted compared to Na (by a factor of 100-300), which is surprising since these elements have similar abundances in most meteoritic minerals. Also, the Ca abundance peaks in the summer which is opposite to the layers of Na and Fe, and the abundance ratio of Ca+ ions to Ca is about,2 whereas the Na+/Na and Fe+/Fe ratios are between 0.1 and 0.2. Furthermore, calcium, along with the other meteoric metals, is of interest since metals in the atmosphere are involved in various phenomena such as sporadic E layers, sporadic metal layers and meteor smoke and it has been suggested that metals are also connected to noctilucent clouds and the Junge layer in the stratosphere.2 Since only Ca and Ca+ can be observed directly in the atmosphere using lidar, laboratory studies and modelling are necessary to understand the chemistry controlling the Ca layer. The two experimental techniques used to measure the relevant rate coefficients in our laboratory are pulsed laser photolysis / laser induced fluorescence (PLP/LIF) and a fast flow tube.
Calcium Ion Chemistry
PLP-LIF Apparatus
Ca+ was produced from pulsed ArF excimer laser photolysis of a precursor, Ca(TMHD)2, at 193 nm. The Ca+ ions were probed at 393.4 nm using a frequency-tripled Nd-YAG pumped dye laser. The reaction took place in a chamber, as shown in the diagram below, which has two pairs of orthogonal, horizontal side-arms and a vertical sidearm on the top.4 Ca(TMHD)2 was heated in one of the side-arms, then the vapour is entrained in He bath gas and carried to the reaction chamber. The reaction mixture entered the chamber via several of the side-arms. A photomultiplier tube attached to the vertical side-arm measureed the LIF signal, from which the pseudo-first order rate coefficient was determined. The following reactions have been measured using this technique:4,5
Ca+ + N2O → CaO+ + N2
Ca+ + O3 → CaO+ + O2
Ca+ + O2 + He → Ca.O2+ + He
Ca+ + N2 + He → Ca.N2+ + He
Ca+ + H2O + He → Ca.H2O+ + He
Ca+ + CO2 + He → Ca.CO2+ + He
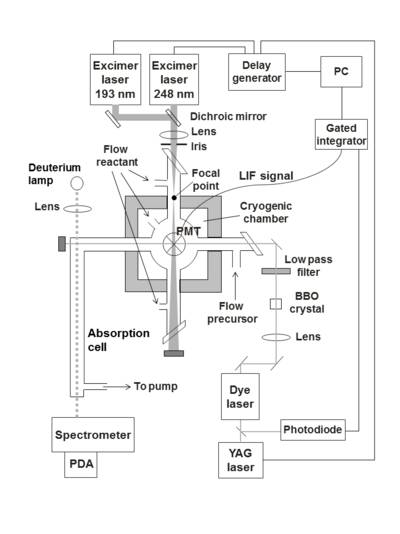 Schematic diagram showing the PLP-LIF apparatus
Schematic diagram showing the PLP-LIF apparatus
Fast flow tube
Ca+ was produced by the pulsed ablation of calcite by an Nd-YAG laser. The Ca+ ions travel down carried by a He bath gas flow and the reactants are added further downstream. Either the loss of Ca+ or production of product ions was monitored with a quadrupole mass spectrometer. This technique has the advantage that successive reactants can be added along the tube, allowing a wider range of reactions to be studied than in the PLP-LIF apparatus. The following ligand-switching reactions were studied:
Ca.CO2+ + H2O → Ca.H2O+ + CO2
Ca.CO2+ + O2 → Ca.O2+ + CO2
Ca.H2O+ + O2 → Ca.O2+ + H2O
For these reactions the rate coefficients were determined using a computer model of the flow tube, which accounts for gas-phase chemistry and diffusion and loss to the walls. The reactions of CaO+ with CO and O and the reaction between CaO2+ and O were also studied using this method.
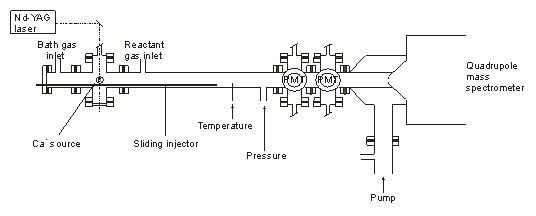 Schematic diagram showing the Fast Flow tube apparatus
Schematic diagram showing the Fast Flow tube apparatus
 Fast flow tube set up during a experiment
Fast flow tube set up during a experiment
Calcium Neutral Chemistry
Ca atoms were produced by the vaporisation of Ca pellets in a high temperature oven and detected using LIF at the downstream end of the flow tube at 422.7 nm. CaO was also monitored by off-resonance LIF (pumped at 385.9 nm, detected at (λ > 693 nm). Reactions studied using this method were:
CaO + O → Ca + O2
CaO2 + O → CaO + O2
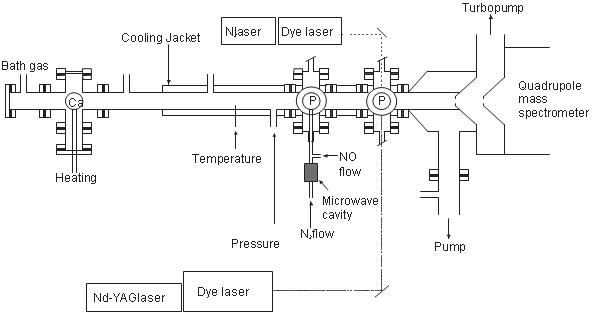
Schematic diagram showing the Fast Flow tube apparatus used for neutrals
References
- Gerding, M., et al., Atmospheric Ca and Ca+ layers: Mid-latitude observations and modelling, J. Geophys. Res., 2000, 105, 27131-27146.
- Plane, J. M. C., Atmospheric Chemistry of Meteoric Metals, Chem. Rev., 2003, 103, 4963-4984.503-512.
- Plane, J. M. C., et al., Kinetic study of the reaction Ca+ + N2O from 188 to 1207 K, J. Phys. Chem. A., 2006, 110, 7874-7881.
- Plane, J. M. C., et al., Kinetic study of the reaction Ca+ + N2O from 188 to 1207 K, J. Phys. Chem. A., 2006, 110, 7874-7881.
- Broadley, S.; Vondrak, T. and Plane, J. M. C., A kinetic study of the reactions of Ca+ ions with O3, O2, N2, CO2 and H2O, PCCP, 2007, 9, 4337-4369.
- Vondrak, T.; Woodcock, K. R. I. and Plane, J. M. C., A kinetic study of the reactions of Fe+ with N2O, N2, O2, CO2 and H2O, and the ligand-switching reactions Fe+.X + Y → Fe+.Y + X (X = N2, O2, CO2; Y = O2, H2O), PCCP, 2006, 8,
