Magnesium Chemistry in the Mesosphere/Lower Thermosphere (MLT)
Middle/Upper Atmosphere Laboratory, Past Research. 2008-2011.
Introduction
Each day approximately 120 tonnes of extra terrestrial material is deposited into the Earth’s upper atmosphere.1 This material undergoes frictional heating when it collides with atmospheric molecules causing metallic species to ablate from the meteoroids surface. The ablated neutral atoms undergo charge transfer with the dominant ionic species in the upper mesosphere/lower thermosphere (MLT), those being O2+ and NO+, producing singly-charged metallic species. These neutral and ionic metal atoms then form metallic layers in the MLT at altitudes between 75 and 110 km. There are many metallic species in the MLT, with the two most abundant being Fe+ and Mg+. The lidar (laser radar) technique has been used extensively to observe these metallic layers, with studies being done on Na, K, Li, Fe, Ca and Ca+.2 However neither Mg nor Mg+ can be observed using lidar as their resonance transitions are in the UV region, below 300 nm, which is strongly absorbed by stratospheric O3. This has led to the study of magnesium relying on rocket-borne mass spectrometers,3, 4 and more recently satellite observations.3, 5, 6 These observations have provided an insight into magnesium in the MLT and highlighted a few interesting characteristics. A striking difference between magnesium and other meteoric metals is the large ratio between Mg+ and Mg. This ratio has been observed to be as low as 3.37 and as high as 228 and 30.9 Magnesium seems to be the only metal displaying such a large ion/neutral ratio. Na+/Na and Fe+/Fe display ratios of ~ 0.23, 4 and Ca+/Ca has a ratio of ~ 2.10 This is even more striking as Mg+ is not significantly depleted relative to other metals in the MLT. The Na+/Mg+ ratio is ~ 0.1 (cosmic abundance 0.05) and the Fe+/Mg+ ratio is ~ 1 (cosmic abundance 0.8).3 Therefore the depletion of Mg is unlikely to be caused by differential ablation. One explanation is that there are substantial differences between the atmospheric chemistries of Mg, Na and Fe. For example the very large Mg+/Mg ratio, compared to other metals, could indicate that Mg+ ions have a long lifetime or that Mg is efficiently removed into a permanent sink below 90 km.
Below is a proposed reaction scheme for atmospheric magnesium.
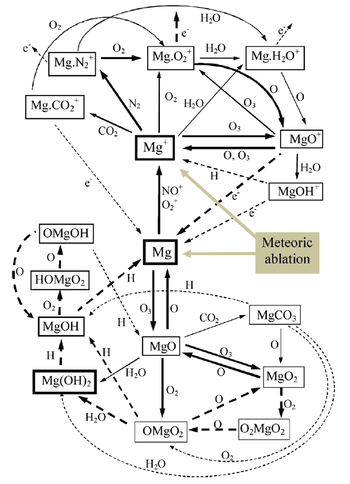
Nonetheless observations alone cannot provide the answers for this peculiar behaviour and this is where laboratory work is needed. Current work uses a pulsed-laser photolysis / laser-induced fluorescence (PLP/LIF) system to measure the rate coefficients for the reactions below.
Mg+ + N2O → MgO+ + N2
Mg+ + O3 → MgO+ + O2
Mg+ + O2 + He → Mg.O2+ + He
Mg+ + H2O + He → Mg.H2O+ + He
Mg+ + CO2 + He → Mg.CO2+ + He
PLP/LIF apparatus
Mg+ was produced from a precursor, Mg(AcAc)2, using a pulsed ArF excimer laser operating at 193 nm. The Mg+ ions were probed at 279.6 nm using a frequency-doubled nitrogen-pumped dye laser. The reaction chamber is shown in the diagram below. The apparatus has two pairs of orthogonal, horizontal side-arms and a vertical sidearm on the top. Mg(AcAc)2 was heated in one of the side-arms, then the vapour was entrained in He bath gas and carried to the reaction chamber where it was photolysed by the excimer laser. The reaction mixture also entered the chamber via several of the side-arms. A photomultiplier tube situated on top of the vertical side-arm measured the LIF signal and from these decays a pseudo first-order rate coefficient was obtained.11, 12
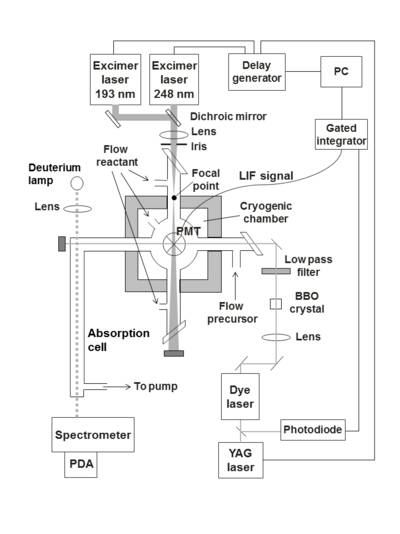
PLP-LIF apparatus
Results
These studies used a combination of laboratory experiments,theory, and atmospheric modeling to develop a better understanding of the chemistry of magnesium in the MLT. The new model is able to reproduce satisfactorily some of the key features of the Mg and Mg+ layers, including the heights of the layers, the seasonal variations of their column abundances, and the unusually large Mg+/Mg ratio. One significant
finding in the study is the role of O2 in recombining with radical species such as MgO2 and MgOH by initiating holding cycles that enhance the ability of these species to act as reservoirs.
The study demonstrated the limits of exploring the kinetics of complex reaction systems using LIF probes, which tend to be restricted to observations of one or two species. Currently we are exploring the use of photoionization time-of-flight mass spectrometry to provide a more general detection system for these metal-containing molecules.
References
- Love, S. G. and Brownlee,D. E. Science, 1993, 262, 550-553.
- Plane, J. M. C. Int. Rev. Phys. Chem., 1991, 10, 55-106.
- Grebowsky, J. M. and Aikin, A. C., in Meteors in the Earth's Atmosphere, ed. M. E. a. W. I. P., Cambridge University Press, Cambridge, Editon edn., 2002.
- Kopp, E., J. Geophys. Res. [Space Phys.], 1997, 102, 9667-9674.
- Gardner, J. A., et al., in Atmospheric Tidal Dynamics and E- and D-Region Physics, Editon edn., 1998, vol. 21, pp. 867-870.
- Gardner J. A., et al., Geophys. Res. Lett., 1995, 22, 2119-2122.
- Aikin, A. C., Grebowsky, J. M. and Burrows, J. P. , in Impact of Minor Bodies of Our Solar System on Planets and Their Middle and Upper Atmosphere, Editon edn., 2004, vol. 33, pp. 1481-1485.
- J. G. Anderson and C. A. Barth, J. Geophys. Res., 1971, 76, 3723.
- Viereck, R. A., et al., in Thermosphere-Ionosphere-Middle Atmosphere Coupling and Dynamics, Editon edn., 1995, vol. 18, pp. 61-64.
- Gerding, M., et al., J. Geophy. Res. [Space Phys.], 2000, 105, 27131-27146.
- Plane, J. M. C. and Whalley, C. L., A New Model for Magnesium Chemistry in the Upper Atmosphere. J. Phys. Chem. A., 2012, 116, 6240-6252.
- Whalley, C.L., et al., A kinetic study of Mg+ and Mg-containing ions reacting with O3, O2, N2, CO2, N2O and H2O: implications for magnesium ion chemistry in the upper atmosphere, PCCP, 2011, 13 (13), 6352-6364.
